![]() 细胞培养基质 层粘连蛋白511
细胞培养基质 层粘连蛋白511
iMatrix-511
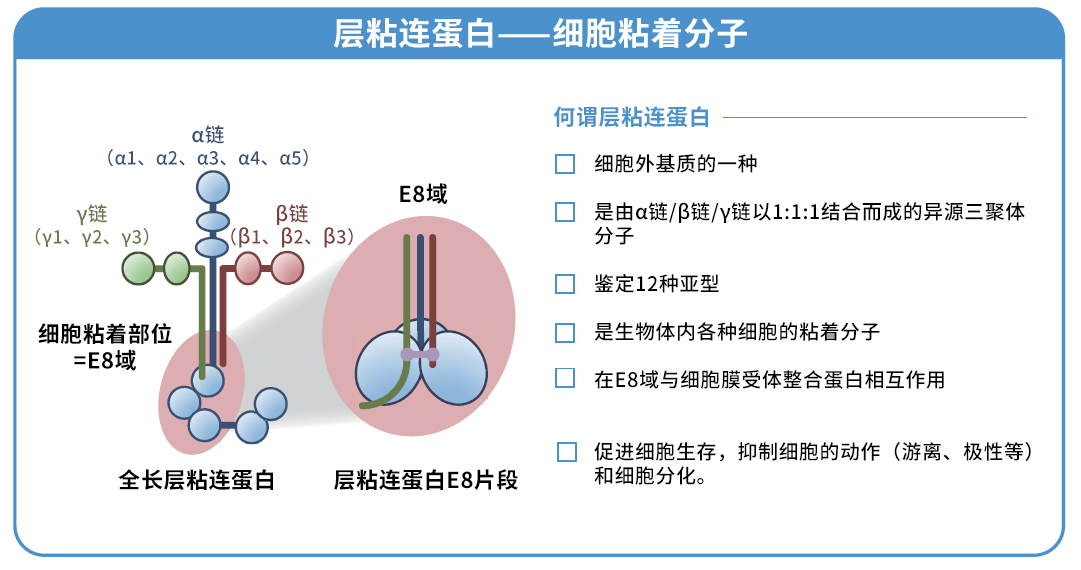
◆什么是层粘连蛋白511?
层粘连蛋白是存在于动物基底膜的一种细胞外基质,已知其与细胞粘附和增殖息息相关。本产品是与层粘连蛋白 511-E8 片段有同一序列的重组蛋白,是可以促进各种细胞粘附和伸展的培养基质。
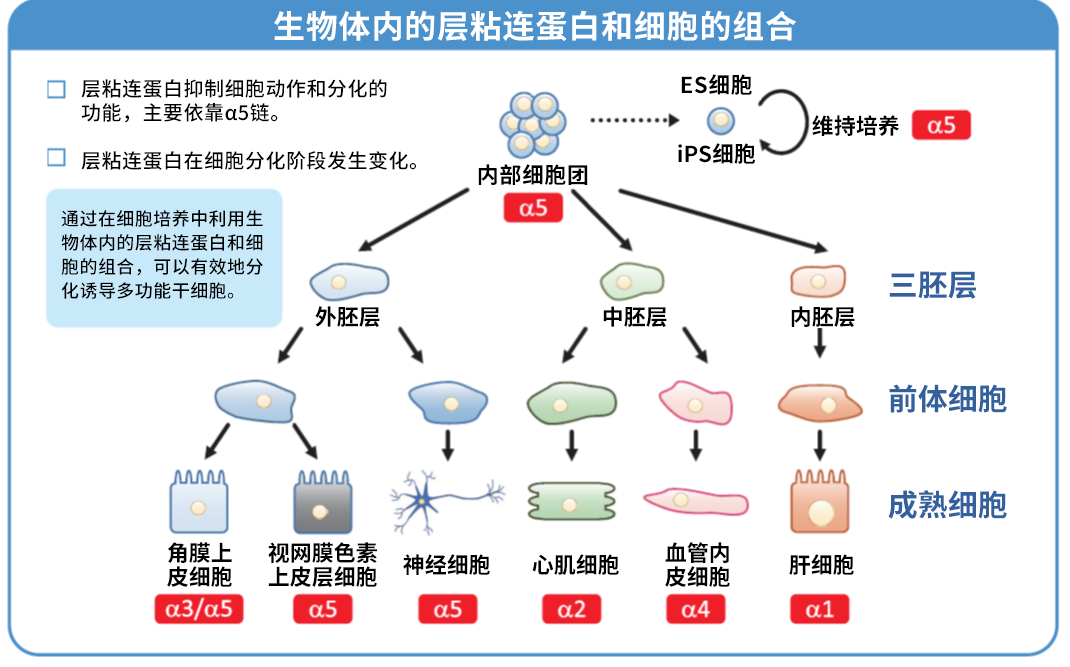
大阪大学和京都大学的共同研究表明,使用iMatrix-511在操作难度非常大的人 iPS 细胞和人 ES 细胞培养中,也可以安全且高效地进行细胞培养。
● 细胞培养的准备非常简单!
● 可用于多种类型的细胞培养!
● 无论分离细胞的状态如何,可实现细胞的高生存率和细胞增殖的高效性!
● 重组蛋白(CHO-S细胞来源),所以混入杂质的危险性低!
● 溶液型试剂,无需溶解,稀释后可直接使用
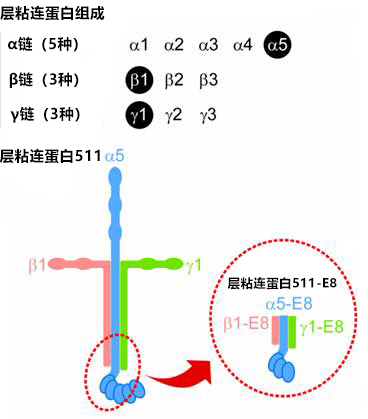
层粘连蛋白511 是由 α5 链、β1 链和 γ1 链组成的层粘连蛋白。层粘连蛋白 511-E8 是层粘连蛋白片段,但其具有与层粘连蛋白全长分子相同的 α6β1 整合蛋白连接功能。
本产品是 Nippi 根据大阪大学和京都大学的专利技术生产贩卖的。
◆使用方法
用 PBS(-)稀释本产品,按照 0.1~1.5 μg/cm2 加入细胞培养器皿中。
※由于细胞种类和细胞株的不同、使用的培养基种类不同,添加的最适剂量会有差异,初次使用时,按照 0.5 μg/cm2 添加培养容器中,逐渐
※调整至最佳使用浓度。
↓
室温下孵育 3 小时,然后去掉溶液。
↓
添加细胞和培养液,进行细胞培养。
● 培养 ES/iPS 细胞时,可进行无饲养层和单细胞继代培养
◆使用案例
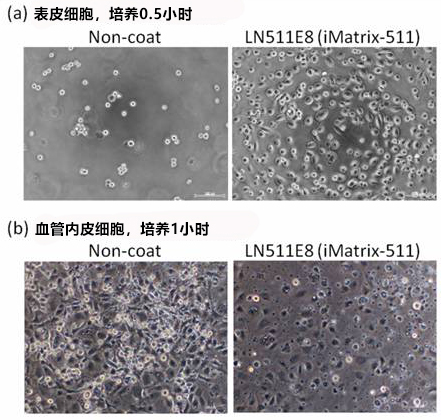
使用本产品对表皮细胞培养 0.5 小时,对血管内皮细胞培养 1 小时。
(a) 表皮细胞,培养 0.5 小时
左(无涂层):大部分细胞未贴壁。
右(iMatrix):多数细胞贴壁并成伸展状态。
(b) 血管内皮细胞,培养 1 小时
左(无涂层):有贴壁的细胞,但大部分多为圆形。
右(iMatrix):较少观察到圆形细胞,几乎所有的细胞表现出了很好的伸展性。
实验人员: (株) Nippi BioMatrix 研究所 藤崎
◆iMatrix-511 与 iMatrix-511 silk 的区别
iMatrix-511 | iMatrix-511 silk | |
生产系统 | 转基因 CHO-S 细胞 | 转基因蚕生产系统 |
提纯材料 | CHO-S 细胞培养上清 | 蚕蛹蛋白 |
产品等级 | 实验研究用* *有临床用级别 | 实验研究用 |
导入基因 | 人层粘连蛋白 511-E8 片段 | |
纯度 | 95% 以上 | |
浓度 | 0.5 mg/mL | |
解离常数 | 10 nM 以下 | |
使用期限 | 生产后 2 年内 | |
iPS 细胞培养能力 | 添加 0.5 μg/cm2 到培养容器中,可用于 iPS 细胞的维持培养 | |
点击此处,选择页面中的”iMatrix™ Calculator“计算实验中 iMatrix-511 使用量
点击此处查看文献应用实例
点击此处查看产品单页:iMatrix系列单页
点击此处查看相关产品页面:iMatrix-411
点击此处查看相关产品页面:iMatrix-332
点击此处查看相关产品页面:iMatrix-221
点击此处查看产品页面:iMatrix-111
iMatrix-511,iMatrix-511 silk
Q1. 产品是以什么样的状态销售的?
A1. 产品是以液态销售的。一支试管里密封装有 0.5 mg/mL 浓度的 175 μg 的细胞培养外基质(层粘连蛋白 511-E8 片段)。
※iMatrix-511 的冻干产品已于2015年3月停止生产。
Q2. 产品的存储条件和有效期是什么?
A2. 产品的储存条件为,冷藏保存在 2-15°C。(推荐 4°C)
A2. 产品的有效期,请参考下表。
产品 | 保质期 |
iMatrix-511 | 自生产后两年内 |
iMatrix-511silk | 自生产后两年内 |
iMatrix-511MG | 自生产后两年内 |
※具体的有效期详见产品外包装。
Q3. 可以冷冻保存吗?
A3. 不可以冷冻保存。
Q4. 产品的纯度是多少?
A4. 纯度为 95% 以上。
Q5. 培养 hES/hiPS 细胞时使用什么培养基最好?
A5. 宫崎等的论文(Nature communications, 3(1236), 1-10, 2012)、(Scientific Reports, 7, 41165, 2017)中,使用了以下的培养基。
・mTeSR1,TeSR2,TeSR-E8 (STEMCELL Technologies)
・Stem Pro hESC SFM (Thermo Fisher Scientific)
・StemFit AK03 (Ajinomoto)
中川等的论文(Scientific Reports, 4(3594), 1-7, 2014)中使用了以下的培养基。
・StemFit (Ajinomoto)
文献中使用的培养基都出现了良好的结果。
Q6. 培养 iPS 细胞时,最佳的涂层浓度是多少?
A6. 最佳的涂层浓度根据细胞株的不同也会有所不同。
A6. 最初请从浓度 0.5 μg/cm2 开始尝试,请根据您使用的细胞株在浓度 0.1~1.5 μg/ cm2 之间考虑。
A6. 另外,还有文献报道了新的不以涂层包被的添加法。
A6. Miyazaki et al. Scientific Reports, 7, 41165, (2017)
Q7. 请教我使用 iMatrix-511 时,培养 iPS 细胞的步骤。
A7. 使用 iMatrix-511 时,ES/iPS 细胞的扩增培养步骤,传代操作,请参考以下的链接。
Q8. 培养 iPS 细胞时,需要 Rock Inhibitor(Y-27632)吗?
A8. 在中川等的论文(Scientific Reports, 4(3594), 2014)中,介绍了只有在传代时添加 Rock Inhibitor,更换培养基的时候不需使用。
Q9. 培养 iPS 细胞时可以单细胞传代吗?
A9. 可以。
※使用 iMatrix-511 时,ES/iPS 细胞的扩增培养步骤,传代操作,请参考以下的链接。
也可以参考中川等的论文(Scientific Reports, 4(3594), 2014)。
Q10. 传代时使用什么细胞分离液?
A10. 可使用胰蛋白酶。
※使用 iMatrix-511 时,ES/iPS 细胞的扩增培养步骤,传代操作,请参考以下的链接。
也可以参考中川等的论文(Scientific Reports, 4(3594), 2014)。
Q11. 可以使用小鼠的 iPS 细胞吗?
A11. 由于没有小鼠 iPS 细胞的培养数据,所以无法回答。
Q12. 这个产品与基质胶有什么不同?
A12. 基质胶含有小鼠EHS肉瘤来源的层粘连蛋白-111。另外还含有层粘连蛋白以外的分子。
A12. iMatrix-511 是将在 CHO-S 细胞中表达的层粘连蛋白 511-E8 片段高度纯化后的重组蛋白。
A12.iMatrix-511silk 是从蚕结的茧中高纯度纯化层粘连蛋白 511-E8 片段的重组蛋白。
A12.已知人ES细胞和 iPS 细胞是通过细胞膜受体(特别是 α6β1整联蛋白)粘附于层粘连蛋白-511。
A12.已知人ES细胞和 iPS 细胞对层粘连蛋白-511 具有高粘附活性。这使得用 iMatrix-511/iMatrix-511silk 可以使 iPS 细胞在单细胞状态下传代。
A12.在宫崎等的论文中(Nature communications, 3(1236), 1-10, 201),5 次传代后(30 天后)的细胞数扩增效率约为基质胶的 200 倍。
参考文献
分类 | 文献信息 | 主题 |
人多能干细胞(hPSC)的确立 | Miyazaki et al. Nat. Commun.3:1236, (2012) | 证实用作hPSC的培养基质的有效性 |
Nakagawa et al. Sci. Rep. 4:3594, (2014) | 确立医疗等级的hPSC | |
Takashima et al. Cell.158(6):1254-69, (2014) | 促进向hPSC的基质状态的转移 | |
Miyazaki et al. Sci. Rep.7:41165, (2017) | 采用无需涂层操作的添加法培养hPSC | |
Sekine et al. Stem Cell Res.24:40-43, (2017) | 确立疾病特异性的hPSC | |
Tan et al. Stem Cell Res. 24:12-15, (2017) | ||
Ishida et al. Sci. Rep. 8(1), 310, (2018) | 利用hPSC的基因编辑建立遗传性疾病模型 | |
Kim et al. Nature Communications, 9(1), 939, (2018) | ||
Sakai-Takemura et al. Sci. Rep, 8, 6555, (2018) | 悬浮培养由hPSC分化的肌肉前体细胞 | |
由hPSC分化衍生的细胞 | Doi et al. Stem Cell Reports. 2(3):337-50, (2014) | 多巴胺产生神经元 |
Ishikawa et al. Hum. Mol. Genet.25(23): 5188-5197, (2016) | ||
Nishimura et al. Stem Cell Reports.6(4): 511-524, (2016) | ||
Samata et al. Nat. Commun. 7:13097, (2016) | ||
Kikuchi et al. Nature. 548(7669):592-596, (2017) | ||
Morizane et al. Nat. Commun.8(1):385, (2017) | ||
Kikuchi et al. J. Neurosci. Res.95(9):1829-37, (2017) | ||
Goparaju et al. Sci. Rep. 7:42367, (2017) | 运动神经元 | |
Burridge et al. Nat. Methods.11(8):855-60, (2014) | 心肌细胞 | |
Sougawa et al. Sci. Rep,8(1), 3726, (2018) | ||
Yamauchi et al. BBRC, 495(1), 1278-1284, (2018) | 心室肌细胞 | |
Akiyama et al. Sci. Rep, 8(1), 1189, (2018) | 骨骼肌细胞 | |
Saito et al. Stem Cell Res Ther, 9(1), 12, (2018) | 成骨细胞 | |
Uchimura et al. Stem cell research, 25, 98-106, (2017) | 成肌细胞 | |
Hayashi et al. Nature.531(7594):376-80, (2016) | 视觉细胞 | |
Hayashi et al. Nat. Protoc.12(4):683-696, (2017) | 角膜上皮细胞 | |
Takayama et al. BBRC. 474(1):91-96, (2016) | 胆管上皮细胞 | |
Takayama et al. Hepatol Commun,1(10), 1058-1069, (2017) | 肝实质细胞 | |
Takayama et al. Biomaterials, (2018) | ||
Takebe et al. Cell Reports, 21(10), 2661-2670, (2017) | 肝细胞 | |
Tan et al. Stem Cell Reports, 11:1-11, (2018) | ||
Camp et al. Nature. 546(7659):533-38, (2017) | 定形内胚层细胞 | |
Zhang et al. Stem Cell Reports, 10(2), 1–14, (2018) | 后内胚层前体细胞 | |
Tanigawa et al. Cell reports, 15(4), 801-813, (2016) | 肾单位前体细胞(胎肾细胞) | |
Musah et al. Nat.Biomed.Eng.1:0069, (2017) | 肾小球上皮细胞 | |
Musah et al. Nature protocols,13(7):1662, (2018) | ||
Mae et al. BBRC, 495(1), 954-961, (2018) | 输尿管芽组织 | |
Oshima et al. BBRC, 497(2), 719-725, (2018) | 血细胞・血管内皮常见前体细胞 | |
Taguchi et al. Cell Stem Cell, 21, (2017) | *培养hPSC用于分化肾单位前体细胞(胎肾细胞) | |
Kawamura et al. Stem Cell Reports.6(3):312-20,(2016) | *培养hPSC用于分化心肌细胞 | |
Sasaki et al. Cell Stem Cell.17(2):178-94, (2015) | *培养hPSC用于分化生殖细胞 | |
Kojima et al. Cell Stem Cell.21(4):517-532, (2017) | ||
Furuta et al. PLoS One. 9(12):e112291, (2014) | *培养hPSC用于分化间充质细胞 | |
人原代细胞的培养 | Okumura et al. Invest. Ophth. Vis. Sci.56(5):2933-42, (2015) | 人角膜内皮细胞 |
Hongo et al. Invest. Ophth. Vis. Sci.58(9):3325-34, (2017) | ||
Polisetti et al. Sci. Rep.7(1):5152, (2017) | 人角膜边缘上皮前体细胞 | |
Ishii et al. Stem Cell Reports, 10, 1-15, (2018) | 卫星细胞 | |
层粘连蛋白-整合素相互作用的分子机制 | Ido et al. J. Biol. Chem. 282(15): 11144-54, (2007) | |
Ido et al. J. Biol. Chem.283(42): 28149-57, (2008) | ||
Taniguchi et al. J. Biol. Chem. 284(12): 7820-31, (2009) | ||
Taniguchi et al. BBRC.487(3): 525-531, (2017) | ||
Takizawa et al. Sci Adv.3(9) :e1701497, (2017) |
英文论文
[1] | Ayabe, H., Anada, T., Kamoya, T., Sato, T., Kimura, M., Yoshizawa, E., Kikuchi, Shunyuu., Ueno, Yasuharu., Sekine, keisuke., J. Gray Camp., Treutlein, B., Ferguson, Autumn., Suzuki, Osamu., Takede, Takanori.. Optimal Hypoxia Regulates Human iPSC-Derived Liver Bud Differentiation through Intercellular TGFB Signaling. Stem Cell Reports, 11, 1-11, (2018)
|
[2] | Musah, S., Dimitrakakis, N., Camacho, D. M., Church, G. M., Ingber, D. E.. Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a lomerulus Chip. Nature protocols, 13(7), 1662, (2018)
|
[3] | Ishii, K., Sakurai, H., Suzuki, N., Mabuchi, Y., Sekiya, I., Sekiguchi, K., Akazawa, C.. Recapitulation of Extracellular LAMININ Environment Maintains Stemness of Satellite Cells In Vitro. Stem Cell Reports, 10, 1-15, (2018)
|
[4] | Ishida, K., Xu, H., Sasakawa, N., Lung, M. S. Y., Kudryashev, J. A., Gee, P., & Hotta, A.. Site-specific randomization of the endogenous genome by a regulatable CRISPR-Cas9 piggyBac system in human cells. Scientific Reports, 8(1), 310, (2018)
|
[5] | Takayama, K., Hagihara, Y., Toba, Y., Sekiguchi, K., Sakurai, F., Mizuguchi, H.. Enrichment of high- functioning human iPS cell-derived hepatocyte-like cells for pharmaceutical research. Biomaterials, (2018)
|
[6] | Akiyama, T., Sato, S., Chikazawa-Nohtomi, N., Soma, A., Kimura, H., Wakabayashi, S., Ko, S.B., Ko, M. S.. Efficient differentiation of human pluripotent stem cells into skeletal muscle cells by combining RNA-based MYOD1-expression and POU5F1-silencing. Scientific Reports, 8(1), 1189, (2018)
|
[7] | Saito, A., Ooki, A., Nakamura, T., Onodera, S., Hayashi, K., Hasegawa, D., Okudaira,T., Watanabe, K., Kato, H., Onda, T., Watanabe, A., Kosaki, K., Nishimura, K., Ohtaka, Manami., Nakanishi, M., Sakamoto, T., Yamaguchi, A., Sueishi, K., Azuma, T.. Targeted reversion of induced pluripotent stem cells from patients with human cleidocranial dysplasia improves bone regeneration in a rat calvarial bone defect model. Stem Cell Research & Therapy, 9(1), 12, (2018)
|
[8] | Yamauchi, K., Li, J., Morikawa, K., Liu, L., Shirayoshi, Y., Nakatsuji, N., Elliott, A. D., Hisatome, I., Suemori, H..Isolation and characterization of ventricular-like cells derived from NKX2-5 eGFP/w and MLC2v mCherry/w double knock-in human pluripotent stem cells. Biochemical and Biophysical Research Communications, 495(1), 1278-1284, (2018)
|
[9] | Mae, S., Ryosaka, M., Toyoda, T., Matsuse, K., Oshima, Y., Tsujimoto, H., Okumura, S., Shibasaki, A., Osafune, K.. Generation of branching ureteric bud tissues from human pluripotent stem cells. Biochemical and biophysical research communications, 495(1), 954-961, (2018)
|
[10] | Kagihiro, M., Fukumori, K., Aoki, T., Ungkulpasvich, U., Mizutani, M., Viravaidya-Pasuwat, K.,& Kino-oka, M.. Kinetic analysis of cell decay during the filling process: Application to lot size determination in manufacturing systems for human induced pluripotent and mesenchymal stem cells. Biochemical Engineering Journal, 131, 31-38, (2018)
|
[11] | Zhang, R. R., Koido, M., Tadokoro, T., Ouchi, R., Matsuno, T., Ueno, Y., Sekine, K., Takebe, T., Taniguchi, H.. Human iPSC-Derived Posterior Gut Progenitors Are Expandable and Capable of Forming Gut and Liver Organoids. Stem Cell Reports, 10(2), 1?14, (2018)
|
[12] | Oshima, K., Saiki, N., Tanaka, M., Imamura, H., Niwa, A., Tanimura, A., Nagahashi, A., Hirayama, A., Okitac, K., Hotta, A., Kitayama, S., Osawa, M., Kaneko, S., Watanabe, A., Asaka, I., Fujibuchi, W., Imai, K., Yabe, H., Kamachi, Y., Hara, J., Kojima, S., Tomita, M., Soga, T., Noma, T., Nonoyama, S., Nakahata, T., Saito, MK.. Human AK2 links intracellular bioenergetic redistribution to the fate of hematopoietic progenitors. Biochemical and Biophysical Research Communications, 497(2), 719- 725, (2018)
|
[13] | Sougawa, N., Miyagawa, S., Fukushima, S., Kawamura, A., Yokoyama, J., Ito, E., Harada, A., Okimoto, K., Mochisuki-Oda, N., Saito, A., Sawa, Y.. Immunologic targeting of CD30 eliminates tumourigenic human pluripotent stem cells, allowing safer clinical application of hiPSC-based cell therapy. Scientific Reports, 8(1), 3726, (2018)
|
[14] | Yasuda, S. Y., Ikeda, T., Shahsavarani, H., Yoshida, N., Nayer, B., Hino, M., Vartak-Sharma, N., Suemori, H., Hasegawa, K.. Chemically defined and growth-factor-free culture system for the expansion and derivation of human pluripotent stem cells. Nature Biomedical Engineering, 2(3), 173, (2018)
|
[15] | Kim, S. I., Matsumoto, T., Kagawa, H., Nakamura, M., Hirohata, R., Ueno, A., Ohishi, M., Sakuma, T., Soga, T., Yamamoto, T., Woltjen, K.. Microhomology-assisted scarless genome editing in human iPSCs. Nature Communications, 9(1), 939, (2018)
|
[16] | Hayashi, R., Ishikawa, Y., Katori, R., Sasamoto, Y., Taniwaki, Y., Takayanagi, Tsujikawa, M., Sekiguchi, K., Quantock, A. J., Nishida, K. . Coordinated generation of multiple ocular-like cell lineages and fabrication of functional corneal epithelial cell sheets from human iPS cells. Nature Protocols, 12(4), 683-696, (2017)
|
[17] | Kikuchi, T., Morizane, A., Okita, K., Nakagawa, M., Yamakado, H., Inoue, H., Takahashi, R., Takahashi, J. . Idiopathic Parkinson's disease patient‐derived induced pluripotent stem cells function as midbrain dopaminergic neurons in rodent brains. Journal of Neuroscience Research, 95(9),1829-37, (2017)
|
[18] | Miyazaki, T., Isobe, T., Nakatsuji, N., & Suemori, H. . Efficient Adhesion Culture of Human Pluripotent Stem Cells Using Laminin Fragments in an Uncoated Manner. Scientific Reports, 7 (41165), 1-8, (2017)
|
[19] | Goparaju, S. K., Kohda, K., Ibata, K., Soma, A., Nakatake, Y., Akiyama, T., Wakabayashi, S., Matsushita, M., Sakota, M., Kimura, H., Yuzaki, M., Shigeru B. H. Ko & Minoru S. H. Ko. . Rapid differentiation of human pluripotent stem cells into functional neurons by mRNAs encoding transcription factors. Scientific Reports, 7, 42367, (2017)
|
[20] | Musah, S., Mammoto, A., Ferrante, C. T., Jeanty, S.S., Hirano-Kobayashi, M., Mammoto, T., Roberts, K., Chung, S., Novak, R., Ingram, M., Fatanat-Didar, T., Koshy, S., Weaver, C. J., Church, M. G., Ingber, F. D. . Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nature Biomedical Engineering, 1 (0069), (2017)
|
[21] | Camp, J. G., Sekine, K., Gerber, T., Loeffler-Wirth, H., Binder, H., Gac, M., Kanton, S., Kageyama, J., Damm, G., Seehofer, D., Belicova, L., Barsacchi, M., Barsacchi, R., Okuda, R., Yoshizawa, E., Kimura, M., Ayabe, H., Taniguchi, H., Takebe, T., & Belicova, L.. Multilineage communication regulates human liver bud development from pluripotency. Nature, 546, 533-538, (2017)
|
[22] | Polisetti, N., Sorokin, L., Okumura, N., Koizumi, N., Kinoshita, S., Kruse, F. E., and Schlotzer- Schrehardt, U. Laminin-511 and-521-based matrices for efficient ex vivo-expansion of human limbal epithelial progenitor cells. Scientific Reports, 7, 5152, (2017)
|
[23] | Hongo, A., Okumura, N., Nakahara, M., Kay, E. P., & Koizumi, N.. The Effect of a p38 Mitogen- Activated Protein Kinase Inhibitor on Cellular Senescence of Cultivated Human Corneal Endothelial CellsEffect of a p38 MAPK Inhibitor on Corneal Endothelial Cells. Investigative Ophthalmology & Visual Science, 58(9), 3325-3334, (2017)
|
[24] | Taniguchi, Y., Li, S., Takizawa, M., Oonishi, E., Toga, J., Yagi, E., & Sekiguchi, K. Probing the acidic residue within the integrin binding site of laminin-511 that interacts with the metal ion- dependent adhesion site of α6β1 integrin. Biochemical and Biophysical Research Communications, 487(3), 525-531, (2017)
|
[25] | Sekine, S. I., Kondo, T., Murakami, N., Imamura, K., Enami, T., Shibukawa, R., Tsukita, K., Funayama, M., Inden, M., Kurita, H., Hozumi, I., Inoue, H.. Induced pluripotent stem cells derived from a patient with familial idiopathic basal ganglia calcification (IBGC) caused by a variant in SLC20A2 gene. Stem Cell Research, (2017)
|
[26] | Tan, G. W., Kondo, T., Murakami, N., Imamura, K., Enami, T., Tsukita, K., Shibukawa, R., Funayama, M., Matsumoto, R., Ikeda, I., Takahashi, R., Inoue, H.. Induced pluripotent stem cells derived from an autosomal dominant lateral temporal epilepsy (ADLTE) patient carrying S473L mutation in leucine-rich glioma inactivated 1 (LGI1). Stem Cell Research, (2017)
|
[27] | Sato-Nishiuchi, R., Li, S., Ebisu, F., Sekiguchi, K.. Recombinant laminin fragments endowed with collagen-binding activity: A tool for conferring laminin-like cell-adhesive activity to collagen matrices. Matrix Biology, (2017)
|
[28] | Kikuchi, T., Morizane, A., Doi, D., Magotani, H., Onoe, H., Hayashi, T., Mizuma, H., Takara, S., Takahashi, R., Inoue, H., Morita, S., Yamamoto, M., Okita, K., Nakagawa, M., Parmar, M., Takahashi, J.. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson's disease model. Nature, 548, 592-596, (2017)
|
[29] | Takizawa, M., Arimori, T., Taniguchi, Y., Kitago, Y., Yamashita, E., Takagi, J., Sekiguchi, K.. Mechanistic basis for the recognition of laminin-511 by α6β1 integrin. Science Advances, 3(9), e1701497, (2017)
|
[30] | Morizane, A., Kikuchi, T., Hayashi, T., Mizuma, H., Takara, S., Doi, H., Mawatari, A., Glasser, M.F., Shiina, T., Ishigaki, H., Itoh, Y., Okita, K., Yamasaki, E., Doi, D., Onoe, H., Ogasawara, K., Yamanaka, S., and Takahashi, J. . MHC matching improves engraftment of iPSC-derived neurons in non- human primates. Nature Communications, 8(1), 385, (2017)
|
[31] | Kikuchi, T., Morizane, A., Doi, D., Magotani, H., Onoe, H., Hayashi, T., Mizuma, H., Takara, S., Takahashi, R., Inoue, H., Morita, S., Yamamoto, M., Okita, K., Nakagawa, M., Parmar, M., Takahashi, J. . human ips cell-derived dopaminergic neurons function in a primate Parkinson's disease model. Nature, 548(7669), 592-596, (2017)
|
[32] | Kojima, Y., Sasaki, K., Yokobayashi, S., Sakai, Y., Nakamura, T., Yabuta, Y., Nakaki, F., Nagaoka, S., Woltjen, K., Hotta, A., Yamamoto, T., Saitou, M.. Evolutionarily Distinctive Transcriptional and Signaling Programs Drive Human Germ Cell Lineage Specification from Pluripotent Stem Cells. Cell Stem Cell, 21(4), 517-532.e5, (2017)
|
[33] | Taguchi, A., & Nishinakamura, R.. Higher-Order Kidney Organogenesis from Pluripotent Stem Cells. Cell Stem Cell, 21. (2017)
|
[34] | Takebe, T., Sekine, K., Kimura, M., Yoshizawa, E., Ayano, S., Koido, M., Funayama, S., Nakanishi, N., Hisai, T., Kobayashi, T., Kasai, T., Kitada, R., Mori, A., Ayabe, H., Ejiri, Y., Amimoto, N., Yamazaki, Y., Ogawa, S., Ishikawa, M., Kiyota, Y., Sato, Y., Nozawa, K., Okamoto, S., Ueno, Y., Kasai, T.. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Reports, 21(10), 2661-2670, (2017)
|
[35] | Uchimura, T., Otomo, J., Sato, M., Sakurai, H.. A human iPS cell myogenic differentiation system permitting high-throughput drug screening. Stem cell research, 25, 98-106, (2017)
|
[36] | Sougawa, N., Miyagawa, S., Fukushima, S., Saito, A., Yokoyama, J., Kitahara, M., Harada, A., Sato- Nishiuchi, R., Sekiguchi, K., Sawa, Y.. Novel Stem Cell Niches Laminin 511 Promotes Functional Angiogenesis Through Enhanced Stem Cell Homing by Modulating" Stem Cell Beds" in the Failed Heart.Circulation, 136(1), A15587, (2017)
|
[37] | Samata, B., Doi, D., Nishimura, K., Kikuchi, T., Watanabe, A., Sakamoto, Y., Kakuta, J., Ono, Y.,& Takahashi, J.. Purification of functional human ES and iPSC-derived midbrain dopaminergic progenitors using LRTM1. Nature Communications, 7(13097), 1-11, (2016)
|
[38] | Hayashi, R., Ishikawa, Y., Sasamoto, Y., Katori, R., Nomura, N., Ichikawa, T., Araki, S., Soma, T., Kawasaki, S., Sekiguchi, K., Tsujikawa, M., Nishida, K., & Quantock, A. J.. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature, 531(7594), 376-380, (2016)
|
[39] | Matsuno, K., Mae, S. I., Okada, C., Nakamura, M., Watanabe, A., Toyoda, T., Uchida, E., Osafune, K.. Redefining definitive endoderm subtypes by robust induction of human induced pluripotent stem cells.Differentiation; research in biological diversity, (2016)
|
[40] | Nishimura, K., Doi, D., Samata, B., Murayama, S., Tahara, T., Onoe, H., & Takahashi, J.. Estradiol Facilitates Functional Integration of iPSC-Derived Dopaminergic Neurons into Striatal Neuronal Circuits via Activation of Integrin α5β1. Stem cell reports, 6(4), 511-524, (2016)
|
[41] | Takayama, K., Mitani, S., Nagamoto, Y., Sakurai, F., Tachibana, M., Taniguchi, Y., Sekiguchi,K., Mizuguchi, H.. Laminin 411 and 511 promote the cholangiocyte differentiation of human induced pluripotent stem cells. Biochemical and biophysical research communications, 474(1), 91-96, (2016)
|
[42] | Kawamura, T., Miyagawa, S., Fukushima, S., Maeda, A., Kashiyama, N., Kawamura, A., Miki, K., Okita, K., Yoshida, Y., Shiina, T., Ogasawara, K., Miyagawa, S., Toda, K., Okuyama, H., Sawa,Y.. Cardiomyocytes derived from MHC-homozygous induced pluripotent stem cells exhibit reduced allogeneic immunogenicity in MHC-matched non-human primates. Stem cell reports, 6(3), 312- 320, (2016).
|
[43] | Tanigawa, S., Taguchi, A., Sharma, N., Perantoni, A. O., & Nishinakamura, R.. Selective in vitro propagation of nephron progenitors derived from embryos and pluripotent stem cells. Cell reports, 15(4), 801-813, (2016)
|
[44] | Okumura, N., Kakutani, K., Numata, R., Nakahara, M., Schlotzer-Schrehardt, U., Kruse, F., Kinoshita. K., Koizumi, N.. Laminin-511 and-521 Enable Efficient In Vitro Expansion of Human Corneal Endothelial CellsLaminin-511 and-521 Enable Expansion of HCECs. Investigative ophthalmology & visual science, 56(5), 2933-2942, (2015)
|
[45] | Sasaki, K., Yokobayashi, S., Nakamura, T., Okamoto, I., Yabuta, Y., Kurimoto, K., Ohta, H., Moritoki, Y., Iwatani, C., Tsuchiya, H., Nakamura, S., Sekiguchi, K., Sakuma, T., Yamamoto, T., Mori, T., Woltjen, K., Nakagawa, M., Yamamoto, T., Takahashi, K., Yamanaka, S., Saitou, M.. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell stem cell, 17(2), 178-194, (2015)
|
[46] | Nakagawa, M., Taniguchi, Y., Senda, S., Takizawa, N., Ichisaka, T., Asano, K., Morizane, A., Doi, D., Takahashi, J., Nishizawa, M., Yoshida, Y., Toyoda, T., Osafune, K., Sekiguchi, K., & Yamanaka, S. . A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Scientific reports, 4(3594), 1-7, (2014)
|
[47] | Doi, D., Samata, B., Katsukawa, M., Kikuchi, T., Morizane, A., Ono, Y., Sekiguchi, K., Nakagawa, M., Parmar, M., Takahashi, J.. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem cell reports, 2(3), 337-350, (2014)
|
[48] | Takashima, Y., Guo, G., Loos, R., Nichols, J., Ficz, G., Krueger, F., Oxley, D., Santos, F., Clarke, J., Mansfield, W., Reik, W., Bertone, P., Smith, A.. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell, 158(6), 1254-1269, (2014)
|
[49] | Fukuta, M., Nakai, Y., Kirino, K., Nakagawa, M., Sekiguchi, K., Nagata, S., Matsumoto, Y., Yamamoto, T., Umeda, K., Heike, T., Okumura, N., Koizumi, N., Sato, T., Nakahata, T., Saito, M., Otsuka, T., Kinoshita, S., Ueno, M., Ikeya, M., Toguchida, J. . Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PloS one, 9(12), e112291, (2014)
|
[50] | Burridge, P. W., Matsa, E., Shukla, P., Lin, Z. C., Churko, J. M., Ebert, A. D., Lan, F., Diecke, S., Huber, B., Mordwinkin, N. M., Plews, J. R., Abilez, O. J., Cui, B., Gold, J. D., & Wu, J. C. . Chemically defined generation of human cardiomyocytes. Nature methods, 11(8), 855-860, (2014)
|
[51] | Miyazaki, T., Futaki, S., Suemori, H., Taniguchi, Y., Yamada, M., Kawasaki, M., Hayashi, M., Kumagai, H., Nakatsuji, N., Sekiguchi, K., & Kawase, E. . Laminin E8 fragment support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nature communications, 3(1236), 1-10, (2012)
|
[52] | Taniguchi, Y., Ido, H., Sanzen, N., Hayashi, M., Sato-Nishiuchi, R., Futaki, S., & Sekiguchi, K. . The C-terminal region of laminin β chains modulates the integrin binding affinities of laminins. Journal of Biological Chemistry, 284(12), 7820-7831, (2009)
|
[53] | Ido, H., Nakamura, A., Kobayashi, R., Ito, S., Li, S., Futaki, S., & Sekiguchi, K. . The requirement of the glutamic acid residue at the third position from the carboxyl termini of the laminin γ chains in integrin binding by laminins. Journal of Biological Chemistry, 282(15), 11144-11154, (2007) |
| 产品编号 | 产品名称 | 产品规格 | 产品等级 | 备注 |
| 385-07361 | iMatrix-511 solution(0.5 mg/mL) 层粘连蛋白511-E8片段,溶液(0.5 mg/mL) |
175 µg×2 | - | - |
| 381-07363 | iMatrix-511 solution(0.5 mg/mL) 层粘连蛋白511-E8片段,溶液(0.5 mg/mL) |
175 µg×6 | - | - |
| 387-10131 | iMatrix-511 Silk 层粘连蛋白511 Silk |
175 µg×6 | - | - |
| 免责声明 |
|
1. 本公司密切关注本网站发布的内容,但不保证发布内容的准确性、完整性、可靠性和最新性等。 2. 本公司不保证使用本网站期间不会出现故障或计算机病毒污染的风险。 3. 无论何种原因,使用本网站时给用户或第三方造成的任何不利或损害,本公司概不负责。此外,对于用户与其他用户或第三方之间因本网站发生的任何交易、通讯 3. 或纠纷,本公司概不负责。 4. 本网站可提供的所有产品和服务均不得用于人体或动物的临床诊断或治疗,仅可用于科研等非医疗目的。如任何用户将本网站提供的产品和服务用于临床诊断或治 4. 疗,以及其他特定的用途或行为,本公司概不保证其安全性和有效性,并且不负任何相关的法律责任。 |