用于通过 SDS-PAGE 分离磷酸化蛋白质
Phos-tag® Acrylamide
仅需将Phos-tag® Acrylamide与丙烯酰胺溶液混合并聚合,即可生成用于分离磷酸化和非磷酸化蛋白质的Phos-tag® SDS-PAGE。
在不使用放射性同位素的情况下,利用 Phos-tag™ SDS-PAGE 即可分离不同条带中的磷酸化和非磷酸化蛋白。分离后的凝胶可用于 Western blotting 和质谱分析等后续实验。
Phos-tag™ SDS-PAGE 操作简单,只需在常规 SDS-PAGE 胶中加入 Phos-tag™ Acrylamide 和 Mn2+ 或者 Zn2+ 即可进行实验。在电泳过程中,磷酸化蛋白的磷酸基团与 Phos-tag™ 中的二价金属离子相结合,降低其迁移速度,从而可区分磷酸化与非磷酸化蛋白。

◆优点、特色
● 采用 Phos-tag™ SDS-PAGE 可轻松分离磷酸化蛋白
无任何放射性元素及化学标记!
● 不论氨基酸残基的类型/位点如何,均可使用
可用于未知的磷酸化分析
可用于检测新的磷酸化位点(无需购买商品化磷酸化抗体、无需特意制备磷酸化抗体)
● 磷酸化位点数量/位点不同的磷酸形式也可分离
显示磷酸化程度和磷酸化形式
● 可同时检测磷酸化/非磷酸化形式
可定量各种磷酸化形式
可简单明确有无磷酸化
● 无需RI或特殊的仪器
实验成本低,操作简单。(SDS-PAGE的试剂→只要有设备即可使用)
● 电泳后可用于WB、MS分析,也可用于二维电泳
WB:可分析内源性蛋白
MS:可显示各磷酸化形式的磷酸化位点的组合
二维电泳:可分离相同等电点和分子量的磷酸化形式
适用于内源性蛋白的磷酸化分析!
◆Phos-tag® SDS-PAGE的原理
Phos-tag® Acrylamide的结构
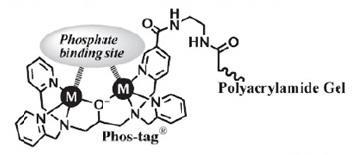
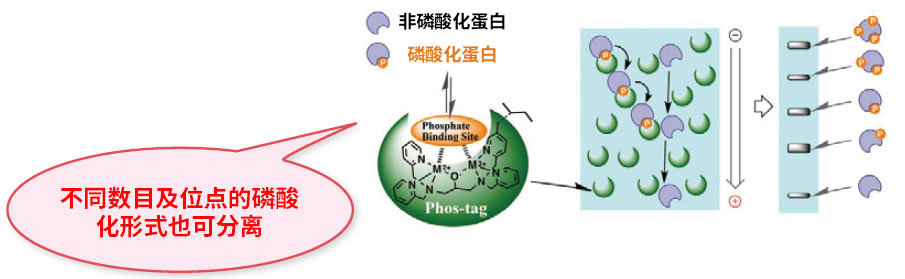
1. 电泳中的磷酸化蛋白会被2个二价金属离子捕获
2. 磷酸化水平越高,电泳速度越慢
3. 根据磷酸化水平进行分离(即使磷酸化位点的数量相同,只要位置不同也可进行分离)
常规的SDS-PAGE(上图:非Phos-tag® 条件下)中,无法确认底物是否被磷酸化。
◆案例、应用
【使用Phos-tag™ SDS-PAGE的磷酸化/非磷酸化蛋白比较】
“我推荐使用Phos-tag ™ ——东京大学研究院医学研究科 小川觉之”
Phos-tag ™ 是专为研究磷酸化蛋白而新开发出来的试剂。此产品使用方便,不但可用于体外实验,还能定量分析体内蛋白的磷酸化水平。Phos-tag ™ SDS-PAGE 可用于常规电泳实验,无需购买特殊设备,性价比高。传统蛋白磷酸化的研究需要特异的磷酸化抗体、RI 等其它试剂,操作复杂,花费大,且放射性元素会有安全隐患,而 Phos-tag ™ 的出现恰恰可以弥补这些缺点,为磷酸化蛋白研究提供新的方向。
磷酸化蛋白和非磷酸化蛋白利用Phos-tag ™ SDS-PAGE 的分离效果图
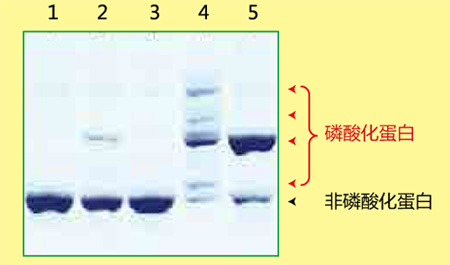
Lane 1 为非磷酸化蛋白,Lane 2-5 为磷酸化蛋白,各蛋白因磷酸化状态不同而条带迁移率也有所不同。
磷酸化/ 非磷酸化蛋白的数量比、磷酸化程度、磷酸化蛋白的丰度等都可根据条带迁移和条带浓度求得。
【资料提供】
日本东京大学研究生院医学系研究科
【二维电泳中的应用:分析 hnRNP K 磷酸化异构体】
小鼠巨噬细胞 J774.1 经 LPS 刺激后,裂解细胞,经过免疫沉淀法分离得到 hnRNP K 。在二维电泳中,一维是IPG 胶,二维是 Phos-tag ™ SDS-PAGE,可分离 hnRNP K 的异构体。利用质谱仪,可以确认不同的点代表不同的亚型或修饰蛋白。
二维电泳
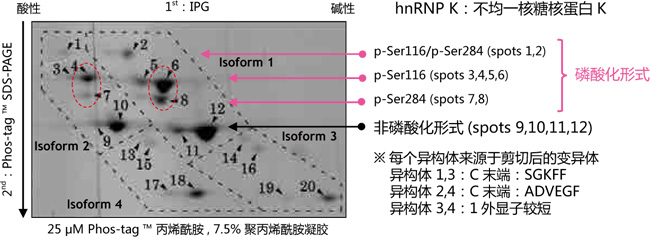
同一个等电点的位置上,不同位点发生磷酸化都可以被区分开来(例: spots 6 vs. 8 and spots 4 vs. 7)
【参考文献】
Characterization of multiple alternative forms of heterogeneous nuclear ribonucleoprotein K by phosphate-affinity electrophoresis. Y. Kimura, K. Nagata, N Suzuki, R. Yokoyama, Y. Yamanaka, H. Kitamura, H. Hirano, and O. Ohara, Proteomics , Nov 2010; 10(21): 3884-95.
【结果提供】
横滨市立大学 生命纳米系统科学研究科 生物体超分子系统科学专业 木村弥生(Dr. Y. Kimura)、平野久(Dr. H. Hirano)理化学研究所 RCAI 小原收
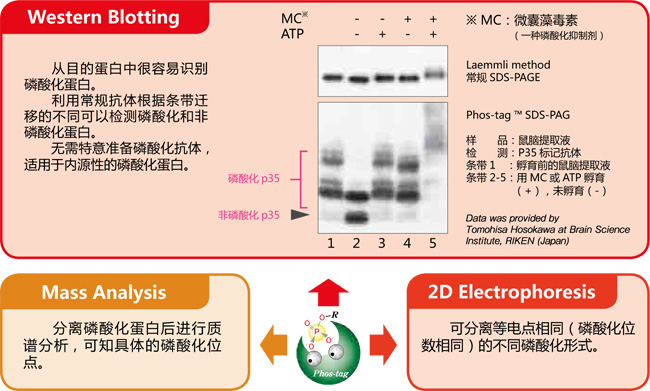
【EGF 刺激前后MAPK 磷酸化水平的变化】
常规 SDS-PAGE 和Phos-tagTM SDS-PAGE 后迚行克疫印迹实验分析 EGF 刺激的 A431 细胞中 MAPK 磷酸化水平。
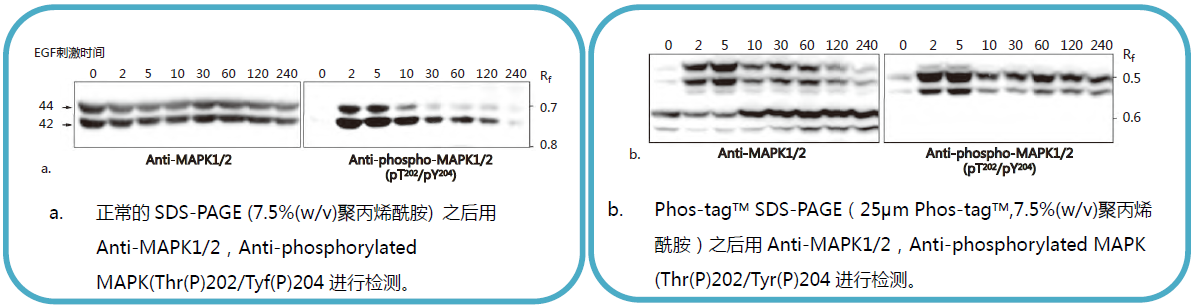
摘自 Kinoshita-Kikuta, E. et al., Mol.Cell. Proteomics. (2007)6: 356.
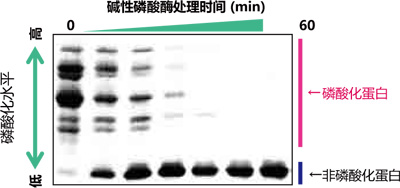
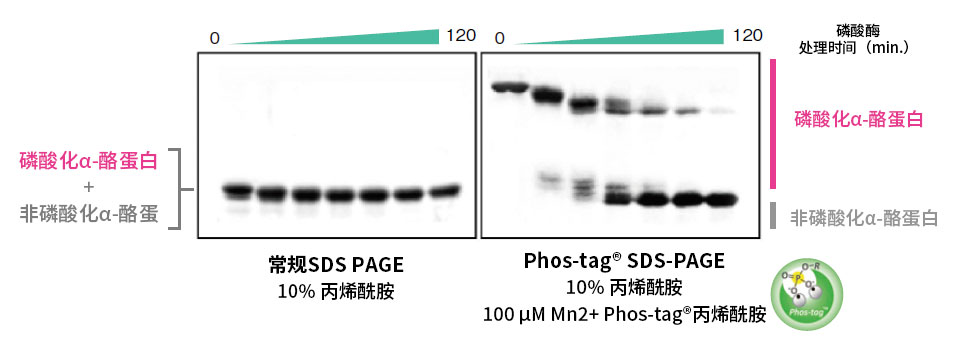
【使用示例:α-酪蛋白去磷酸化反应随时间的变化】
使用Phos-tag® SDS-PAGE和常规SDS-PAGE分离碱性磷酸酶随时间(孵育时间:0~120 min)去磷酸化的α-酪蛋白样品。
◆相关产品
产品名称 | 用 途 |
Phos-tag™ Acrylamide | 分离: SDS - PAGE 分离不同磷酸化水平的蛋白 |
SuperSep Phos-tag™ | 分离: 预制胶中含有50μM Phos-tag™ Acrylamide |
Phos-tag™ Biotin | 检测: 代替 Western Blot 检测中的磷酸化抗体 |
Phos-tag™ Agarose | 纯化: 通用柱层析,纯化磷酸化蛋白 |
Phos-tag™ Mass Analytical Kit | 分析: 用于质谱 MALDI-TOF/MS 分析,提高磷酸化分子的检测灵敏度 |
phos-tag™ 由日本广岛大学研究生院医齿药学综合研究科医药分子功能科学研究室开发,对应指导手册请见相关资料。
操作视频,请点击:
样品处理(TCA沉淀):http://labchem.fujifilm-wako.com.cn/resources/show/47.html
凝胶制备:http://labchem.fujifilm-wako.com.cn/resources/show/48.html
1. Phos-tag® Acrylamide的溶解
5 mmmol/ Phos-tag® 液体 (3v/v% 甲醇):
1) 10 mg Phos-tag® Acrylamide 里加入 0.1 mL 甲醇
2) 使用枪头搅拌混合直至完全溶解。
3) 加 3.2 mL 蒸馏水, 用枪头混匀。
2-8℃ 避光保存。不适合零度以下保存。建议保存时间6个月。
注意:避免溶解过程出现白色悬浮颗粒。
2. α-Casein, from Bovine Milk, Dephosphorylated(038-23221),阳性对照(含有磷酸化和非磷酸化α-Casein),如何使用?
用水或者上样 buffer 溶解。用水溶解后,冷冻保存。电泳条件:Phos-tag® 50 umol/L,分离胶浓度 10% 。
电流:30 mM,1小时。
3. 用Alkaline Phosphatase(for Biochemistry)(018-10693)进行磷酸化蛋白的去磷酸化反应体系。
37℃,过夜。# 10 mg/mL phosphorylated protein 50 μL
# 0.50 M Tris/HCl buffer (pH 9.0) containing 0.10 M MgCl2 10 μL
# Sterilized water 39 μL
# Alkaline phosphatase(018-10693). 0.3 unit / 1 μL 有一点需要注意:ALP 活性化使用 Mg 离子,相同的非磷酸化蛋白质用 ALP
处理的样品的条带和没有用ALP处理的样品的条带的位置不同。
4. Phos-tag® SDS-PAGE 实验没有成功分离磷酸化蛋白:
1) 使用 α-Casein, from Bovine Milk, Dephosphorylated(038-23221)作为阳性对照,确认实验条件和试剂均没有问题。
2) 可使用 Phos-tag® Biotin 检测样品中是否有磷酸化蛋白。确认有磷酸化蛋白后,再通过 Phos-tag® SDS-PAGE 进行分离鉴定。
3) 经质谱鉴定有表达磷酸化蛋白,建议增大样品的含量,可使用 Phos-tag® Agarose 进行磷酸化蛋白的富集。磷酸化蛋白含量过低,
会影响其分离效果。
4) 文献报道有表达磷酸化蛋白,或者同源蛋白有表达磷酸化蛋白的,建议用 Phos-tag® Biotin 先确认样品中是否有磷酸化蛋白。
5) 建议样品的 pH 值在7左右。酸性或者碱性条件下,Mn2+ -Phos-tag® 与磷酸化基团的特异性结合较差。
6) 避免样品中含有高浓度的还原剂,变性剂,表面活性剂等。β-巯基乙醇浓度不高于 0.2 M(或者5%)。
7) 进行 Phos-tag® SDS-PAGE 的最佳样品是纯化的蛋白。如果是细胞裂解液,体外激酶反应液,组织均浆液等,需要摸索最佳的分
离胶,Phos-tag® Acylamide 的浓度。建议 Phos-tag® Acrylamide 浓度从 50 μM 开始摸索。
5. Phos-tag® SDS-PAGE 凝胶用于 Western Blotting 实验的优化建议:
1) 可以检测的样品包括体外激酶反应体系,细胞裂解液,组织均浆液。
2) 每孔样品的上样量是 10~30 μg(请根据蛋白表达量进行调整)
3) 制备样品中含有的还原剂、变性剂、螯合剂、钒酸等会使电泳条带发生弯曲或者拖尾。通过 TCA 沉淀或渗析法降低杂质含量。
4) 建议样品的 pH 值在7左右。如果加入上样缓冲液后溶液显黄色或者橙色,加入 Tris 缓冲液调整 pH 值为7。
5) 目的蛋白分子量大于 60 kDa,分离胶的丙烯酰胺浓度为6%;目的蛋白分子量小于 60 kDa,分离胶的丙烯酰胺浓度为8%。
6) 如果样品中含有大量蛋白,比如细胞裂解液,组织均浆液,Phos-tag® Acylamide 浓度为 5~25 uM。
若目的蛋白浓度低,建议 Phos-tag® Acylamide 浓度为 100 uM。
7) Phos-tag® SDS-PAGE凝胶用于 Western Blotting 实验,湿法转膜建议:10 mM EDTA 的转移缓冲液处理凝胶 10 min,不含有
EDTA 的转移缓冲液处理凝胶 10 min。重复3次。强烈建议湿法转膜。
8) Phos-tag® SDS PAGE 半干法转膜建议:
i. 电泳后用含有 EDTA 的转移缓冲液处理凝胶,EDTA的浓度为 100 mM。100 mM EDTA 的转移缓冲液处理凝胶 10 min,不含有
EDTA的转移缓冲液处理凝胶 10 min。重复3次。
ii. 转膜的电流值提高2%~3%, 延长时间2成。
iii. 转膜的缓冲液加 SDS,加到大约 0.05~0.2%,转膜效率会提高。
9) 使用目的蛋白的非磷酸化抗体即可。比如检测各种肿瘤细胞系中 Src 激酶活性实验,用 Src 的非磷酸化抗体即可。
10) 富士胶片和光的 WIDE-VIEW™ Prestained Protein Siza MarkerIII(230-02461)可检测作为转膜效率,但是无法判断分子量。
11) 一般预染的蛋白 marker 在 Phos-tag® SDS-PAGE 中条带会弯曲,无法判断蛋白分子量。
12) 不能确认磷酸化蛋白和非磷酸化蛋白的分离,请进行常规的 SDS-PAGE,Western Blotting 实验。比对目的蛋白的迁移率。
13) 不能确认是因为蛋白发生磷酸化还是出现降解造成蛋白条带迁移,请进行常规的 SDS-PAGE 实验,确认不会出现条带迁移。
14) 目的蛋白磷酸化与非磷酸化分离效果不佳,使用 α-Casein、from Bovine Milk、Dephosphorylated(038-23221)作为阳性对照,
确认实验条件和试剂均没有问题。如果确认能够分离,调整分离胶,Phos-tag® Acylamide 的浓度。建议使用品质佳的 MnCl2
(139-00722)。
【参考文献】
· Conversion of graded phosphorylation into switch-like nuclear translocation via autoregulatory mechanisms in ERK signalling[J].Nature communications, 2016, 7,Shindo Y, Iwamoto K, Mouri K, et al.
· PTEN modulates EGFR late endocytic trafficking and degradation by dephosphorylating Rab7[J]. Nature communications, 2016, 7,Shinde S R, Maddika S.
· Feedback control of ErbB2 via ERK-mediated phosphorylation of a conserved threonine in the juxtamembrane domain[J]. Scientific Reports, 2016, 6: 31502,Kawasaki Y, Sakimura A, Park C M, et al.
· Plastid-nucleus communication involves calcium-modulated MAPK signalling[J]. Nature Communications, 2016, 7,Guo H, Feng P, Chi W, et al.
· Sequential domain assembly of ribosomal protein S3 drives 40S subunit maturation[J]. Nature communications, 2016, 7,Mitterer V, Murat G, Réty S, et al.
· Phos-tag analysis of Rab10 phosphorylation by LRRK2: a powerful assay for assessing kinase function and inhibitors[J]. Biochemical Journal, 2016: BCJ20160557,Ito G, Katsemonova K, Tonelli F, et al.
· Analysis of phosphorylation of the myosin targeting subunit of smooth muscle myosin light chain phosphatase by Phos-tag SDS-PAGE[J]. The FASEB Journal, 2016, 30(1 Supplement): 1209.1-1209.1,Walsh M P, MacDonald J A, Sutherland C.
· Using Phos-Tag in Western Blotting Analysis to Evaluate Protein Phosphorylation[J]. Kidney Research: Experimental Protocols, 2016: 267-277,Horinouchi T, Terada K, Higashi T, et al.
· The Abundance of Nonphosphorylated Tau in Mouse and Human Tauopathy Brains Revealed by the Use of Phos-Tag Method[J]. The American journal of pathology, 2016, 186(2): 398-409,Kimura T, Hatsuta H, Masuda-Suzukake M, et al.
· Phos-tag SDS-PAGE resolves agonist-and isoform-specific activation patterns for PKD2 and PKD3 in cardiomyocytes and cardiac fibroblasts[J]. Journal of Molecular and Cellular Cardiology, 2016,Qiu W, Steinberg S F.
· Analysis of phosphorylation of the myosin-targeting subunit of myosin light chain phosphatase by Phos-tag SDS-PAGE[J]. American Journal of Physiology-Cell Physiology, 2016, 310(8): C681-C691,Sutherland C, MacDonald J A, Walsh M P.
· Electrochemical biosensor for protein kinase A activity assay based on gold nanoparticles-carbon nanospheres, phos-tag-biotin and β-galactosidase[J]. Biosensors and Bioelectronics, 2016, 86: 508-515,Zhou Y, Yin H, Li X, et al.
· Validation of Cis and Trans Modes in Multistep Phosphotransfer Signaling of Bacterial Tripartite Sensor Kinases by Using Phos-Tag SDS-PAGE[J]. PloS one, 2016, 11(2): e0148294,Kinoshita-Kikuta E, Kinoshita E, Eguchi Y, et al.
· Phosphopeptide Detection with Biotin-Labeled Phos-tag[J]. Phospho-Proteomics: Methods and Protocols, 2016: 17-29,Kinoshita-Kikuta E, Kinoshita E, Koike T.
· A Phos‐tag SDS‐PAGE method that effectively uses phosphoproteomic data for profiling the phosphorylation dynamics of MEK1[J]. Proteomics, 2016,Kinoshita E, Kinoshita‐Kikuta E, Kubota Y, et al.
· Difference gel electrophoresis of phosphoproteome: U.S. Patent Application 15/004,339[P]. 2016-1-22,Tao W A, Wang L.
· ERK1/2-induced phosphorylation of R-Ras GTPases stimulates their oncogenic potential[J]. Oncogene, 2016,Frémin C, Guégan J P, Plutoni C, et al.
· Microtubules Inhibit E-Cadherin Adhesive Activity by Maintaining Phosphorylated p120-Catenin in a Colon Carcinoma Cell Model[J]. PloS one, 2016, 11(2): e0148574,Maiden S L, Petrova Y I, Gumbiner B M.
· Serine 231 and 257 of Agamous-like 15 are phosphorylated in floral receptacles[J]. Plant Signaling & Behavior, 2016, 11(7): e1199314,Patharkar O R, Macken T A, Walker J C.
· A small molecule pyrazolo [3, 4-d] pyrimidinone inhibitor of zipper-interacting protein kinase suppresses calcium sensitization of vascular smooth muscle[J]. Molecular pharmacology, 2016, 89(1): 105-117,MacDonald J A, Sutherland C, Carlson D A, et al.
· The RNA polymerase II C-terminal domain phosphatase-like protein FIERY2/CPL1 interacts with eIF4AIII and is essential for nonsense-mediated mRNA decay in Arabidopsis[J]. The Plant Cell, 2016: TPC2015-00771-RA,Chen T, Qin T, Ding F, et al.
· Vasorelaxant Effect of 5′-Methylthioadenosine Obtained from Candida utilis Yeast Extract through the Suppression of Intracellular Ca2+ Concentration in Isolated Rat Aorta[J]. Journal of agricultural and food chemistry, 2016, 64(17): 3362-3370,Kumrungsee T, Akiyama S, Saiki T, et al.
· Inhibition of deubiquitinating activity of USP14 decreases tyrosine hydroxylase phosphorylated at Ser19 in PC12D cells[J]. Biochemical and biophysical research communications, 2016, 472(4): 598-602,Nakashima A, Ohnuma S, Kodani Y, et al.
· Actin Tyrosine-53-Phosphorylation in Neuronal Maturation and Synaptic Plasticity[J]. The Journal of Neuroscience, 2016, 36(19): 5299-5313,Bertling E, Englund J, Minkeviciene R, et al.
· AMPK-dependent phosphorylation of lipid droplet protein PLIN2 triggers its degradation by CMA[J]. Autophagy, 2016, 12(2): 432-438,Kaushik S, Cuervo A M.
· Myocardin-related transcription factor a and yes-associated protein exert dual control in G protein-coupled receptor-and RhoA-mediated transcriptional regulation and cell proliferation[J]. Molecular and cellular biology, 2016, 36(1): 39-49,Olivia M Y, Miyamoto S, Brown J H.
· Extensive phosphorylation of AMPA receptors in neurons[J]. Proceedings of the National Academy of Sciences, 2016, 113(33): E4920-E4927,Diering G H, Heo S, Hussain N K, et al.
· The transmembrane region of guard cell SLAC1 channels perceives CO2 signals via an ABA-independent pathway in Arabidopsis[J]. The Plant Cell, 2016, 28(2): 557-567,Yamamoto Y, Negi J, Wang C, et al.
· The Hippo pathway mediates inhibition of vascular smooth muscle cell proliferation by cAMP[J]. Journal of molecular and cellular cardiology, 2016, 90: 1-10,Kimura T E, Duggirala A, Smith M C, et al.
· Atg13 is essential for autophagy and cardiac development in mice[J]. Molecular and cellular biology, 2016, 36(4): 585-595,Kaizuka T, Mizushima N.
· The ChrSA and HrrSA two-component systems are required for transcriptional regulation of the hemA promoter in Corynebacterium diphtheriae[J]. Journal of Bacteriology, 2016: JB. 00339-16,Burgos J M, Schmitt M P.
· Intergenic Variable-Number Tandem-Repeat Polymorphism Upstream of rocA Alters Toxin Production and Enhances Virulence in Streptococcus pyogenes[J]. Infection and Immunity, 2016, 84(7): 2086-2093,Zhu L, Olsen R J, Horstmann N, et al.
· Receptor for advanced glycation end products (RAGE) knockout reduces fetal dysmorphogenesis in murine diabetic pregnancy[J]. Reproductive Toxicology, 2016, 62: 62-70,Ejdesjö A, Brings S, Fleming T, et al.
· Aurora kinase-induced phosphorylation excludes transcription factor RUNX from the chromatin to facilitate proper mitotic progression[J]. Proceedings of the National Academy of Sciences, 2016, 113(23): 6490-6495,Chuang L S H, Khor J M, Lai S K, et al.
· Quantitative phosphoproteomics of protein kinase SnRK1 regulated protein phosphorylation in Arabidopsis under submergence[J]. Journal of experimental botany, 2016: erw107,Cho H Y, Wen T N, Wang Y T, et al.
· Temporal regulation of lipin activity diverged to account for differences in mitotic programs[J]. Current Biology, 2016, 26(2): 237-243,Makarova M, Gu Y, Chen J S, et al.
· Block of CDK1‐dependent polyadenosine elongation of Cyclin B mRNA in metaphase‐i‐arrested starfish oocytes is released by intracellular pH elevation upon spawning[J]. Molecular reproduction and development, 2016, 83(1): 79-87,Ochi H, Aoto S, Tachibana K, et al.
· Mitotic Exit Function of Polo-like Kinase Cdc5 Is Dependent on Sequential Activation by Cdk1[J]. Cell reports, 2016, 15(9): 2050-2062,Rodriguez-Rodriguez J A, Moyano Y, Játiva S, et al.
· PLK2 phosphorylates and inhibits enriched TAp73 in human osteosarcoma cells[J]. Cancer medicine, 2016, 5(1): 74-87,Hu Z B, Liao X H, Xu Z Y, et al.
· Phosphorylated TDP-43 becomes resistant to cleavage by calpain: A regulatory role for phosphorylation in TDP-43 pathology of ALS/FTLD[J]. Neuroscience research, 2016, 107: 63-69,Yamashita T, Teramoto S, Kwak S.
· The Pch2 AAA+ ATPase promotes phosphorylation of the Hop1 meiotic checkpoint adaptor in response to synaptonemal complex defects[J]. Nucleic acids research, 2016: gkw506,Herruzo E, Ontoso D, González-Arranz S, et al.
· An optimized guanidination method for large‐scale proteomic studies[J]. Proteomics, 2016,Ye J, Zhang Y, Huang L, et al.
· Expression and purification of the kinase domain of PINK1 in Pichia pastoris[J]. Protein Expression and Purification, 2016,Wu D, Qu L, Fu Y, et al.
· BRI2 and BRI3 are functionally distinct phosphoproteins[J]. Cellular signalling, 2016, 28(1): 130-144,Martins F, Rebelo S, Santos M, et al.
· Identification of glycoproteins associated with HIV latently infected cells using quantitative glycoproteomics[J]. Proteomics, 2016,Yang W, Jackson B, Zhang H.
· Regulation of Beclin 1 Protein Phosphorylation and Autophagy by Protein Phosphatase 2A (PP2A) and Death-associated Protein Kinase 3 (DAPK3)[J]. Journal of Biological Chemistry, 2016, 291(20): 10858-10866,Fujiwara N, Usui T, Ohama T, et al.
· Regulatory Implications of Structural Changes in Tyr201 of the Oxygen Sensor Protein FixL[J]. Biochemistry, 2016, 55(29): 4027-4035,Yamawaki T, Ishikawa H, Mizuno M, et al.
· Histone demethylase Jmjd3 regulates osteoblast apoptosis through targeting anti-apoptotic protein Bcl-2 and pro-apoptotic protein Bim[J]. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 2016, 1863(4): 650-659,Yang D, Okamura H, Teramachi J, et al.
· Analysis of Molecular Species Profiles of Ceramide-1-phosphate and Sphingomyelin Using MALDI-TOF Mass Spectrometry[J]. Lipids, 2016, 51(2): 263-270,Yamashita R, Tabata Y, Iga E, et al.
· Highly sensitive myosin phosphorylation analysis in the renal afferent arteriole[J]. Journal of Smooth Muscle Research, 2016, 52(0): 45-55,Takeya K.
· Functional dissection of the CroRS two-component system required for resistance to cell wall stressors in Enterococcus faecalis[J]. Journal of bacteriology, 2016, 198(8): 1326-1336,Kellogg S L, Kristich C J.
· Regulation of mitogen-activated protein kinase by protein kinase C and mitogen-activated protein kinase phosphatase-1 in vascular smooth muscle[J]. American Journal of Physiology-Cell Physiology, 2016, 310(11): C921-C930,Trappanese D M, Sivilich S, Ets H K, et al.
· ModProt: a database for integrating laboratory and literature data about protein post-translational modifications[J]. Journal of Electrophoresis, 2016, 60(1): 1-4,Kimura Y, Toda T, Hirano H.
· The C-ETS2-TFEB Axis Promotes Neuron Survival under Oxidative Stress by Regulating Lysosome Activity[J]. Oxidative medicine and cellular longevity, 2016,Ma S, Fang Z, Luo W, et al.
· Essential role of the PSI–LHCII supercomplex in photosystem acclimation to light and/or heat conditions by state transitions[J]. Photosynthesis Research, 2016: 1-10,Marutani Y, Yamauchi Y, Higashiyama M, et al.
· Identification of a redox-modulatory interaction between selenoprotein W and 14-3-3 protein[J]. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 2016, 1863(1): 10-18,Jeon Y H, Ko K Y, Lee J H, et al.
· Effects of hydrogen sulfide on the heme coordination structure and catalytic activity of the globin-coupled oxygen sensor AfGcHK[J]. BioMetals, 2016, 29(4): 715-729,Fojtikova V, Bartosova M, Man P, et al.
· Identification and functional analysis of phosphorylation in Newcastle disease virus phosphoprotein[J]. Archives of virology, 2016: 1-14,Qiu X, Zhan Y, Meng C, et al.
· Increased level of phosphorylated desmin and its degradation products in heart failure[J]. Biochemistry and Biophysics Reports, 2016, 6: 54-62,Bouvet M, Dubois-Deruy E, Alayi T D, et al.
· Profiling DNA damage-induced phosphorylation in budding yeast reveals diverse signaling networks[J]. Proceedings of the National Academy of Sciences, 2016: 201602827,Zhou C, Elia A E H, Naylor M L, et al.
· Unexpected properties of sRNA promoters allow feedback control via regulation of a two-component system[J]. Nucleic Acids Research, 2016: gkw642,Brosse A, Korobeinikova A, Gottesman S, et al.
· Evolution of ZnII–Macrocyclic Polyamines to Biological Probes and Supramolecular Assembly[J]. Macrocyclic and Supramolecular Chemistry: How Izatt-Christensen Award Winners Shaped the Field, 2016: 415,Kimura E, Koike T, Aoki S.
· Phosphopeptide Enrichment Using Various Magnetic Nanocomposites: An Overview[J]. Phospho-Proteomics: Methods and Protocols, 2016: 193-209,Batalha Í L, Roque A C A.
· In vivo phosphorylation of a peptide tag for protein purification[J]. Biotechnology letters, 2016, 38(5): 767-772,Goux M, Fateh A, Defontaine A, et al.
· Regulation of cell reversal frequency in Myxococcus xanthus requires the balanced activity of CheY‐like domains in FrzE and FrzZ[J]. Molecular microbiology, 2016,Kaimer C, Zusman D R.
· Elevation of cortical serotonin transporter activity upon peripheral immune challenge is regulated independently of p38 mitogen‐activated protein kinase activation and transporter phosphorylation[J]. Journal of neurochemistry, 2016, 137(3): 423-435,Schwamborn R, Brown E, Haase J.
· The Yeast Cyclin-Dependent Kinase Routes Carbon Fluxes to Fuel Cell Cycle Progression[J]. Molecular cell, 2016, 62(4): 532-545,Ewald J C, Kuehne A, Zamboni N, et al.
· Two Degradation Pathways of the p35 Cdk5 (Cyclin-dependent Kinase) Activation Subunit, Dependent and Independent of Ubiquitination[J]. Journal of Biological Chemistry, 2016, 291(9): 4649-4657,Takasugi T, Minegishi S, Asada A, et al.
· Increased level of phosphorylated desmin and its degradation products in heart failure[J]. Biochemistry and Biophysics Reports. 2016,Bouvet M, Dubois-Deruy E, Alayi T D, et al.
· a high‐affinity LCO‐binding protein of Medicago truncatula, interacts with LYK3, a key symbiotic receptor[J]. FEBS letters, 2016, 590(10): 1477-1487,Fliegmann J, Jauneau A, Pichereaux C, et al. LYR3,
· Nek1 Regulates Rad54 to Orchestrate Homologous Recombination and Replication Fork Stability[J]. Molecular Cell, 2016,Spies J, Waizenegger A, Barton O, et al.
· PhostagTM-gel retardation and in situ thylakoid kinase assay for determination of chloroplast protein phosphorylation targets[J]. Endocytobiosis and Cell Research, 2016, 27(2): 62-70,Dytyuk Y, Flügge F, Czarnecki O, et al.
· Luteinizing Hormone Causes Phosphorylation and Activation of the cGMP Phosphodiesterase PDE5 in Rat Ovarian Follicles, Contributing, Together with PDE1 Activity, to the Resumption of Meiosis[J]. Biology of reproduction, 2016: biolreprod. 115.135897,Egbert J R, Uliasz T F, Shuhaibar L C, et al.
· Newby, AC, & Bond, M.(2016). The Hippo pathway mediates inhibition of vascular smooth muscle cell proliferation by cAMP[J]. Journal of Molecular and Cellular Cardiology, 2016, 90: 1-10,Kimura-Wozniak T, Duggirala A, Smith M C, et al. G.
· Yeast lacking the amphiphysin family protein Rvs167 is sensitive to disruptions in sphingolipid levels[J]. The FEBS Journal, 2016, 283(15): 2911-2928,Toume M, Tani M.
· Regulation of CsrB/C sRNA decay by EIIAGlc of the phosphoenolpyruvate: carbohydrate phosphotransferase system[J]. Molecular microbiology, 2016, 99(4): 627-639,Leng Y, Vakulskas C A, Zere T R, et al.
· The Late S-Phase Transcription Factor Hcm1 Is Regulated through Phosphorylation by the Cell Wall Integrity Checkpoint[J]. Molecular and cellular biology, 2016: MCB. 00952-15,Negishi T, Veis J, Hollenstein D, et al.
· Validation of chemical compound library screening for transcriptional co‐activator with PDZ‐binding motif inhibitors using GFP‐fused transcriptional co‐activator with PDZ‐binding motif[J]. Cancer science, 2016, 107(6): 791-802,Nagashima S, Maruyama J, Kawano S, et al.
· ULK1/2 Constitute a Bifurcate Node Controlling Glucose Metabolic Fluxes in Addition to Autophagy[J]. Molecular cell, 2016, 62(3): 359-370,Li T Y, Sun Y, Liang Y, et al.
· Spatiotemporal dynamics of Oct4 protein localization during preimplantation development in mice[J]. Reproduction, 2016: REP-16-0277,Fukuda A, Mitani A, Miyashita T, et al.
· The tandemly repeated NTPase (NTPDase) from Neospora caninum is a canonical dense granule protein whose RNA expression, protein secretion and phosphorylation coincides with the tachyzoite egress[J]. Parasites & Vectors, 2016, 9(1): 1,Pastor-Fernández I, Regidor-Cerrillo J, Álvarez-García G, et al.
· Interaction Analysis of a Two-Component System Using Nanodiscs[J]. PloS one, 2016, 11(2): e0149187,Hörnschemeyer P, Liss V, Heermann R, et al.
· Constitutive Activation of PINK1 Protein Leads to Proteasome-mediated and Non-apoptotic Cell Death Independently of Mitochondrial Autophagy[J]. Journal of Biological Chemistry, 2016, 291(31): 16162-16174,Akabane S, Matsuzaki K, Yamashita S, et al.
· p38β Mitogen-Activated Protein Kinase Modulates Its Own Basal Activity by Autophosphorylation of the Activating Residue Thr180 and the Inhibitory Residues Thr241 and Ser261[J]. Molecular and cellular biology, 2016, 36(10): 1540-1554,Beenstock J, Melamed D, Mooshayef N, et al.
· Lysophosphatidylcholine acyltransferase 1 protects against cytotoxicity induced by polyunsaturated fatty acids[J]. The FASEB Journal, 2016, 30(5): 2027-2039,Akagi S, Kono N, Ariyama H, et al.
· Characterization of a herpes simplex virus 1 (HSV-1) chimera in which the Us3 protein kinase gene is replaced with the HSV-2 Us3 gene[J]. Journal of virology, 2016, 90(1): 457-473,Shindo K, Kato A, Koyanagi N, et al.
· Generation of phospho‐ubiquitin variants by orthogonal translation reveals codon skipping[J]. FEBS letters, 2016, 590(10): 1530-1542,George S, Aguirre J D, Spratt D E, et al.
· Evolution of KaiC-Dependent Timekeepers: A Proto-circadian Timing Mechanism Confers Adaptive Fitness in the Purple Bacterium Rhodopseudomonas palustris[J]. PLoS Genet, 2016, 12(3): e1005922,Ma P, Mori T, Zhao C, et al.
· Phosphorylation of Bni4 by MAP kinases contributes to septum assembly during yeast cytokinesis[J]. FEMS Yeast Research, 2016, 16(6): fow060,Pérez J, Arcones I, Gómez A, et al.
· Alteration of Antiviral Signalling by Single Nucleotide Polymorphisms (SNPs) of Mitochondrial Antiviral Signalling Protein (MAVS)[J]. PloS one, 2016, 11(3): e0151173,Xing F, Matsumiya T, Hayakari R, et al.
· Arm-in-arm response regulator dimers promote intermolecular signal transduction[J]. Journal of bacteriology, 2016, 198(8): 1218-1229,Baker A W, Satyshur K A, Morales N M, et al.
· The lsh/ddm1 homolog mus-30 is required for genome stability, but not for dna methylation in neurospora crassa[J]. PLoS Genet, 2016, 12(1): e1005790,Basenko E Y, Kamei M, Ji L, et al.
· Fine tuning chloroplast movements through physical interactions between phototropins[J]. Journal of Experimental Botany, 2016: erw265,Sztatelman O, Łabuz J, Hermanowicz P, et al.
· Characterization of the Neospora caninum NcROP40 and NcROP2Fam-1 rhoptry proteins during the tachyzoite lytic cycle[J]. Parasitology, 2016, 143(01): 97-113,Pastor-Fernandez I, Regidor-Cerrillo J, Jimenez-Ruiz E, et al.
· Transcriptional Profile during Deoxycholate-Induced Sporulation in a Clostridium perfringens Isolate Causing Foodborne Illness[J]. Applied and environmental microbiology, 2016, 82(10): 2929-2942,Yasugi M, Okuzaki D, Kuwana R, et al.
· Timely Closure of the Prospore Membrane Requires SPS1 and SPO77 in Saccharomyces cerevisiae[J]. Genetics, 2016: genetics. 115.183939,Paulissen S M, Slubowski C J, Roesner J M, et al.
· DDK dependent regulation of TOP2A at centromeres revealed by a chemical genetics approach[J]. Nucleic Acids Research, 2016: gkw626,Wu K Z L, Wang G N, Fitzgerald J, et al.
· OVATE Family Protein 8 Positively Mediates Brassinosteroid Signaling through Interacting with the GSK3-like Kinase in Rice[J]. PLoS Genet, 2016, 12(6): e1006118,Yang C, Shen W, He Y, et al.
· Epithelial Sel1L is required for the maintenance of intestinal homeostasis[J]. Molecular biology of the cell, 2016, 27(3): 483-490, Sun S, Lourie R, Cohen S B, et al.
· Effect of Sodium Dodecyl Sulfate Concentration on Supramolecular Gel Electrophoresis[J]. ChemNanoMat, 2016,Tazawa S, Kobayashi K, Yamanaka M.
· Intergenic VNTR Polymorphism Upstream of rocA Alters Toxin Production and Enhances Virulence in Streptococcus pyogenes[J]. Infection and immunity, 2016: IAI. 00258-16,Zhu L, Olsen R J, Horstmann N, et al.
· Ajuba Phosphorylation by CDK1 Promotes Cell Proliferation and Tumorigenesis[J]. Journal of Biological Chemistry, 2016: jbc. M116. 722751,Chen X, Stauffer S, Chen Y, et al.
· Editorial: International Plant Proteomics Organization (INPPO) World Congress 2014[J]. Frontiers in Plant Science, 2016, 7,Heazlewood J L, Jorrín-Novo J V, Agrawal G K, et al.
· Phosphoinositide kinase signaling controls ER-PM cross-talk[J]. Molecular biology of the cell, 2016, 27(7): 1170-1180,Omnus D J, Manford A G, Bader J M, et al.
· A multiple covalent crosslinked soft hydrogel for bioseparation[J]. Chemical Communications, 2016, 52(15): 3247-3250,Liu Z, Fan L, Xiao H, et al.
· Advances in crop proteomics: PTMs of proteins under abiotic stress[J]. Proteomics, 2016, 16(5): 847-865,Wu X, Gong F, Cao D, et al.
· Cyclin-Dependent Kinase Co-Ordinates Carbohydrate Metabolism and Cell Cycle in S. cerevisiae[J]. Molecular cell, 2016, 62(4): 546-557,Zhao G, Chen Y, Carey L, et al.
· Carbon Monoxide Gas Is Not Inert, but Global, in Its Consequences for Bacterial Gene Expression, Iron Acquisition, and Antibiotic Resistance[J]. Antioxidants & redox signaling, 2016,Wareham L K, Begg R, Jesse H E, et al.
· Two-layer regulation of PAQR3 on ATG14-linked class III PtdIns3K activation upon glucose starvation[J]. Autophagy, 2016: 1-2,Xu D, Wang Z, Chen Y.
· Regulation of sphingolipid biosynthesis by the morphogenesis checkpoint kinase Swe1[J]. Journal of Biological Chemistry, 2016, 291(5): 2524-2534,Chauhan N, Han G, Somashekarappa N, et al.
· PAX5 tyrosine phosphorylation by SYK co-operatively functions with its serine phosphorylation to cancel the PAX5-dependent repression of BLIMP1: A mechanism for antigen-triggered plasma cell differentiation[J]. Biochemical and biophysical research communications, 2016, 475(2): 176-181,Inagaki Y, Hayakawa F, Hirano D, et al.
· A Combined Computational and Genetic Approach Uncovers Network Interactions of the Cyanobacterial Circadian Clock[J]. Journal of Bacteriology, 2016: JB. 00235-16,Boyd J S, Cheng R R, Paddock M L, et al.
· HuR mediates motility of human bone marrow-derived mesenchymal stem cells triggered by sphingosine 1-phosphate in liver fibrosis[J]. Journal of Molecular Medicine, 2016: 1-14,Chang N, Ge J, Xiu L, et al.
· Combined replacement effects of human modified β-hexosaminidase B and GM2 activator protein on GM2 gangliosidoses fibroblasts[J]. Biochemistry and Biophysics Reports, 2016,Kitakaze K, Tasaki C, Tajima Y, et al.
· Roseotoxin B Improves Allergic Contact Dermatitis through a Unique Anti-inflammatory Mechanism Involving Excessive Activation of Autophagy in Activated T-Lymphocytes[J]. Journal of Investigative Dermatology, 2016,Wang X, Hu C, Wu X, et al.
References on Phos-tag™ Chemistry
· Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of phosphorylated compounds using a novel phosphate capture molecule, Rapid Communications of Mass Spectrometry, 17, 2075-2081 (2003), H. Takeda, A. Kawasaki, M. Takahashi, A. Yamada, and T. Koike
· Recognition of phosphate monoester dianion by an alkoxide-bridged dinuclear zinc (II) complex, Dalton Transactions, 1189-1193 (2004), E. Kinoshita, M. Takahashi, H. Takeda, M. Shiro, and T. Koike
· Quantitative analysis of lysophosphatidic acid by time-of-flight mass spectrometry using a phosphate capture molecule, Journal of Lipid Research, 45, 2145-2150 (2004), T. Tanaka, H. Tsutsui, K. Hirano, T. Koike, A. Tokumura, and K. Satouchi
· Production of 1,2-Didocosahexaenoyl Phosphatidylcholine by Bonito Muscle Lysophosphatidylcholine/Transacylase, Journal of Biochemistry,136, 477-483 (2004), K. Hirano, H. Matsui, T. Tanaka, F. Matsuura, K. Satouchi, and T. Koike
· Novel immobilized zinc(II) affinity chromatography for phosphopeptides and phosphorylated proteins, Journal of Separation Science, 28, 155-162 (2005), E. Kinoshita, A. Yamada, H. Takeda, E. Kinoshita-Kikuta, and T. Koike
| 产品编号 | 产品名称 | 产品规格 | 产品等级 | 备注 |
| 304-93526 | Phos-tag Acrylamide AAL-107 5mM Aqueous Solution Phos-tag 丙烯酰胺5mM水溶液 |
0.3 mL | 蛋白研究 | 溶液型 |
| 300-93523 | Phos-tag Acrylamide AAL-107 Phos-tag 丙烯酰胺 |
2 mg | 蛋白研究 | 粉末型 |
| 304-93521 | Phos-tag Acrylamide AAL-107 Phos-tag 丙烯酰胺 |
10 mg | 蛋白研究 | 粉末型 |
| 产品编号 | 产品名称 | 产品规格 | 产品等级 | 备注 |
| 301-93531 | Phos-tag™ Biotin BTL-104 Phos-tag™ 生物素 |
10 mg | - | - |
| 308-97201 | Phos-tag Biotin BTL-111 1mM Aqueous Solution Phos-tag™ 生物素1mM水溶液 |
0.1 mL | - | - |
| 387-07321 | Phos-tag™ Tip Phos-tag™ 琼脂糖枪头 |
8 pcs | - | - |
| 192-17401 | SuperSep™ Phos-tag™ (50 μmol/L), 6%, 13 well Phos-tag™ 预制胶50 μmol/L,6%,13孔 |
5 块 | 电泳用 | - |
| 199-17391 | SuperSep™ Phos-tag™ (50 μmol/L), 6%, 17 well Phos-tag™ 预制胶50 μmol/L,6%,17孔 |
5 块 | 电泳用 | - |
| 193-16691 | SuperSepTM Phos-tag™ (50 μmol/L), 15%, 13 well Phos-tag™ 预制胶50 μmol/L,15%,13孔 |
5 块 | 电泳用 | - |
| 193-16711 | SuperSep™ Phos-tag™ (50 μmol/L), 10%, 13 well Phos-tag™ 预制胶50 μmol/L,10%,13孔 |
5 块 | 电泳用 | - |
| 196-16701 | SuperSep™ Phos-tag™ (50 μmol/L), 15%, 17 well Phos-tag™ 预制胶50 μmol/L,15%,17孔 |
5 块 | 电泳用 | - |
| 190-16721 | SuperSep™ Phos-tag™ (50 μmol/L), 10%, 17 well Phos-tag™ 预制胶50 μmol/L,10%,17孔 |
5 块 | 电泳用 | - |
| 195-17371 | SuperSep™ Phos-tag™ (50 μmol/L), 7.5%, 13 well Phos-tag™ 预制胶50 μmol/L,7.5%,13孔 |
5 块 | 电泳用 | - |
| 192-17381 | SuperSep™ Phos-tag™ (50 μmol/L), 7.5%, 17 well Phos-tag™ 预制胶50 μmol/L,7.5%,17孔 |
5 块 | 电泳用 | - |
| 193-16571 | SuperSep™ Phos-tag™ (50μmol/L), 12.5%, 17 well Phos-tag™ 预制胶50 μmol/ L,12.5%,17孔(用于Wako EasySeparator电泳仪) |
5 块 | 电泳用 | - |
| 195-16391 | SuperSep™ Phos-tag™ (50μmol/L), 12.5%, 13 well Phos-tag™ 预制胶50μmol/L ,12.5%,13孔 |
5 块 | 电泳用 | - |
| 305-93551 | Phos-tag™ Mass Analytical Kit Phos-tag™ 质谱分析试剂盒 |
1 Set | 电泳用 | - |
| 免责声明 |
|
1. 本公司密切关注本网站发布的内容,但不保证发布内容的准确性、完整性、可靠性和最新性等。 2. 本公司不保证使用本网站期间不会出现故障或计算机病毒污染的风险。 3. 无论何种原因,使用本网站时给用户或第三方造成的任何不利或损害,本公司概不负责。此外,对于用户与其他用户或第三方之间因本网站发生的任何交易、通讯 3. 或纠纷,本公司概不负责。 4. 本网站可提供的所有产品和服务均不得用于人体或动物的临床诊断或治疗,仅可用于科研等非医疗目的。如任何用户将本网站提供的产品和服务用于临床诊断或治 4. 疗,以及其他特定的用途或行为,本公司概不保证其安全性和有效性,并且不负任何相关的法律责任。 |